- Gravimetric Determination Calculation
- Gravimetric Determination Of Chloride Lab
- Gravimetric Determination Of Nickel Dmg In Mac
Experiment 7-023: Gravimetric determination of Nickel in a Five-cent Coin
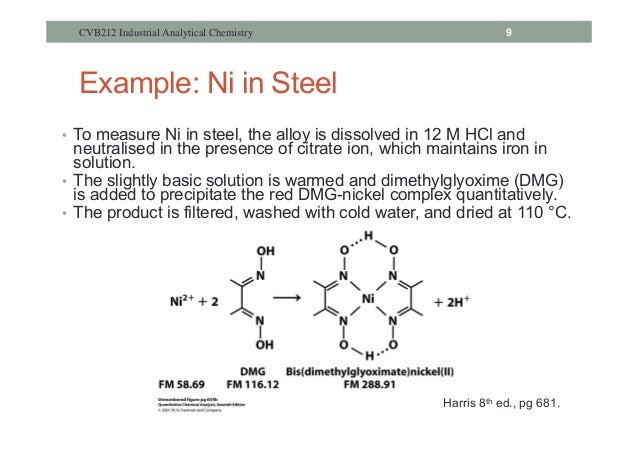
A gravimetric determination of a substance involves the separation of that substance in a form which may be weighed accurately and is of known composition. That usually means that a material dissolved in solution is completely precipitated from solution, and the solid is weighed. In the case of nickel, Ni2+ ions form an extremely insoluble bright red complex with two dimethylglyoxime (DMG) anions under mildly basic conditions. No other common metallic ion reacts in the same way, so the formation of the insoluble Ni(DMG)2 complex makes it possible to separate the Ni2+ ions from other metal ions in solution. The reaction is illustrated below; the dashed lines represent hydrogen bonds which help stabilize the complex.
The Chemistry:
2 Dimethylglyoxime + Ni2+------------> Ni(DMG)2+ 2 H+
The Ni(DMG)2 complex (MW 288.9 g/mole) is stable to heat and is unusually hydrophobic; it will dry quickly in an oven at 110-120 oC to yield a pure substance whose weight can be used to calculate the nickel content of the original sample.
Gravimetric method is used whereby dimethylglyoxime (1% in alcohol) is added to precipitate the analyte which is later separated from the solution and dried. The concentration of the sample solution has been calculated as 0.109 mg/mL. Introduction; In this lab, you will be precipitating nickel (Ni2+) from an unknown Nickel ore. To obtain a gravimetric determination of nickel using dimethylglyoxime: Weigh and measure a sample so that not over 100 mg. Of nickel present. Add 10 cc’s of nitric acid.5 to.1 grams of potassium chlorate and digest on the steam plate adding more potassium chlorate if necessary to effect solution.
A five-cent piece (a nickel) consists of copper alloyed with nickel and other metals. Before the five-cent piece can be analyzed for the nickel content, it must be cleaned, weighed and then dissolved. Reaction of the coin with the nitric acid oxidizes both the nickel and copper atoms and releases various oxides of nitrogen:
3 Cu(s) + 8 H+(aq) + 2 NO3-(aq) -------> 3 Cu2+(aq) + 2 NO(g) + 4 H2O(l) [1]
Determination of nickel(II) as the nickel dimethylglyoxime complex using colorimetric solid phase extraction Article in Analytica Chimica Acta 508(1):53-59 April 2004 with 20,380 Reads. Experiment: Gravimetric Determination of Nickel The purpose of this experiment is to determine the% nickel (by weight) in an unknown nickel-containing ore by means of gravimetric methods. INTRODUCTION The separation of nickel from other ions in a sample is a good example of specificity in quantitative analysis. 1 Experiment No. 1 Determination of Nickel with Dimethylglyoxime (DMG). 1.1 Principle:- This is a gravimetric analysis. Principle behind gravimetric analysis is that the mass of.

3 Ni(s) + 8 H+(aq) + 2 NO3-(aq) --------> 3 Ni2+(aq) + 2 NO(g) + 4 H2O(l) [2]
The resulting solution is acidic and contains both Ni2+ and Cu2+ ions. Before adding the DMG and separating the Ni2+ from the Cu2+ ions, the solution must be neutralized (and made weakly basic) by adding sodium acetate. To avoid interference by Cu2+, sodium tartrate is also added, as the tartrate ion wraps itself around the Cu2+ ions and prevents their precipitation when the DMG is added.
The precipitate (Ni(DMG)2 ) initially produced by adding DMG consists of very small crystals which cannot be filtered, as they penetrate or clog the filter mat. A more tractable precipitate is generated if the solution is simmered for an hour or more to encourage the growth of larger crystals. Such simmering of a precipitate to encourage flocculation (a common technique) is known as digestion. The digested precipitate is filtered and washed extensively with distilled water to remove adsorbed impurities. Extensive washing will not cause a loss of precipitate, as the Ni(DMG)2 complex is extremely insoluble (less than 1.0 mg per liter in cold water).
The Experiment:
This lab requires two weeks to complete, during the first week the nickel samples will be prepared and the Ni(DMG)2 will be precipitated. Between the two weeks, this precipitate will be allowed to digest, so that it can be filtered, dried and weighed during the second week.
Students will work individually, except when preparing the stock sample of nickel (4 students per 5-cent coin).
Week I
Students will pre-dry their crucibles (with filters), prepare the stock sample of nickel and precipitate the Ni(DMG)2 in the first week of this lab.The Ni(DMG)2 samples will be stored in your lab locker and allowed to digest for 1 week.

Week II
Gravimetric Determination Calculation
The Ni(DMG)2 product will be filtered, dried and weighed during week 2. Since there will be a considerable amount of downtime during this week, you will also be working on lab 7-016 while your samples are being filtered and dried (in a drying oven). After allowing your samples to dry and cool, you should mass your samples and determine the % of nickel in a 5-cent coin.
WASTE MANAGEMENT
In this experiment you generate waste containing the toxic metal, nickel. The solid waste Ni(DMG)2 should be placed in the solid chemical waste container and all liquid waste should be placed in the liquid chemical waste container located in the Chemical Waste Hood.
Useful Information:
Calculations: Need some help with calculations?
Gravimetric Determination Of Chloride Lab
Coin Specifications:The U.S. Mint coin specification web page. Check out the rest of the site, it's pretty cool.